How to Keep Perfect Batch Records?
Batch records are documents that detail the journey of raw materials becoming batches of finished goods. Even though the term is mostly associated with process manufacturing, especially the pharma industry, every company that manufactures its products in batches or series can benefit from keeping batch records. Here is how to do it.

You can also listen to this article:
What is a Batch Record?
A batch record is the collection of data related to the manufacturing of a product batch, detailing the processing dates, the lots and quantities of the raw materials used, the staff involved, and the equipment utilized. These records ensure traceability in the supply chain and are therefore essential in quality assurance and regulatory compliance.
Even though batch records are mostly associated with process manufacturing (e.g. pharmaceutical, biotech, food, and chemical industries), discrete manufacturing companies that produce in series can also benefit from the added transparency they provide. Keeping manual batch records, however, could turn into a very challenging task as the complexity of the bills of materials, the number of vendors, and the company itself grows. That is why many manufacturers choose to implement traceability software to create largely automated electronic batch records.
What is an Electronic Batch Record?
An electronic batch record is the product documentation created by manufacturing software with traceability functionality. These types of software allow for automated lot tracking, recording all events related to a stock lot or parts of it.
In a manual system, recording the lot codes and other necessary information at every point of handling can be very time-consuming and a small lot tracking mistake can cascade into much larger problems. Automating these data-entry-based tasks, however, helps minimize the risk of human errors, cut costs, and increase operational efficiency.
In these lot tracking systems, when goods are marked as received, a unique stock lot number is created. When a manufacturing order is due to start and materials are booked from stock, inventory employees can check the system to see which items (i.e. how much, from which location and lot) to pick and transfer to the production floor. Then, when a batch of products is finished and transferred to the warehouse, the system assigns another unique lot number to that batch. When a customer order comes in and products are booked, inventory employees can once again see which lot they have to pick.
This virtual paper trail is the basis for identifying the root causes of issues and complying with modern traceability requirements as every product non-conformity issue can easily be traced back to specific stock lots, workstations, or employees.
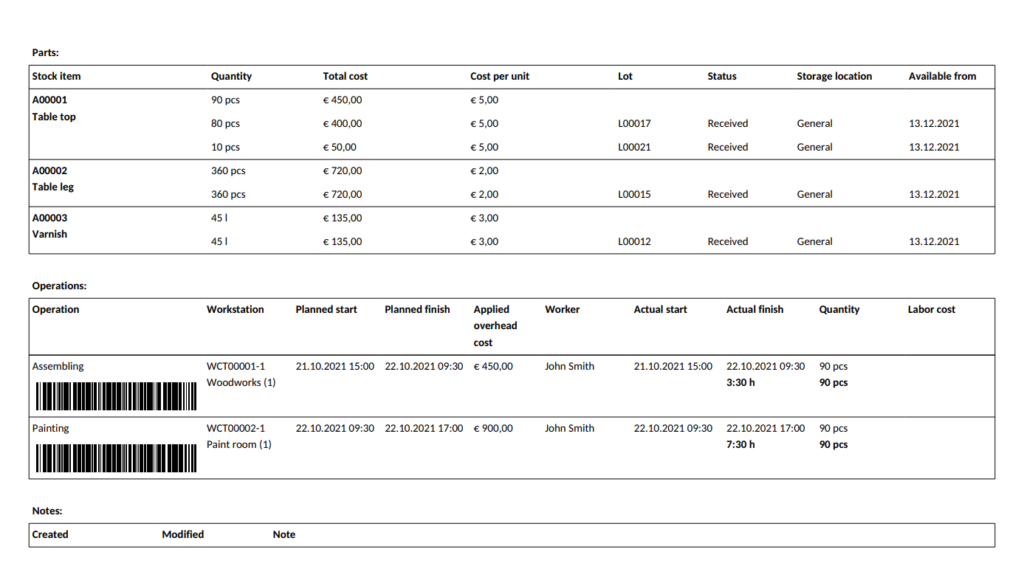
Batch records are often confused with master production records (also called master formula). While batch records detail the material lots, the personnel, the equipment used, and other data related to the handling and production that has already taken place in relation to a batch of products, a master production record is essentially an instruction for manufacturing. It may include the detailed BOM description, equipment list, theoretical and expected yield, and step-by-step instructions for the standard operating procedures to be used during production and inspections.
Tips for keeping perfect Batch Records
As the basic mechanisms of both manual and electronic batch records are the same, we can also use the same general steps for both approaches. Here are some tips for keeping your batch records accurate and organized.
1. Record everything
When health and safety (and regulatory compliance) are concerned, you cannot be thorough enough. That is why you would do well to record each and every instance of when goods are handled, including every inventory movement, each step in the production process, and the inspections the goods pass through.
By recording everything, you make it much easier to detect inconsistencies and pinpoint risk areas where the goods are subject to tampering or damage. Awareness of and transparency within your processes enable you to reduce preventable mistakes, promptly take corrective actions, and improve the quality of your products.
It is highly recommended to use a digital barcode inventory system for recording inventory movements and production events. This would substantially improve the accuracy of your data.
2. Invest in data accuracy
Poor data collection practices always tend to lead to much larger issues throughout the whole company. From being unable to trace back contaminated or faulty batches to simply losing goods within your own facilities, sub-par data accuracy could cost more in the end than investing in immaculate record-keeping.
Allocating resources to data integrity is always very important, but especially so when doing things manually. Common data entry issues such as incorrect values, illegible entries, incomplete information, etc. tend to accumulate and compound over time if they are not quickly resolved. Resolving them, however, requires the installation of control mechanisms such as hiring staff whose task would be to specifically ensure data integrity.
A more cost-effective solution would be to use traceability software with a barcode functionality, which would automate a large portion of these tasks. Of course, data should still be periodically validated, e.g. by conducting regular stocktakes, but it would be a much less resource-heavy task.
3. Organize and integrate the data
All that data collected and controlled needs to be kept organized and easily accessible. Traditionally, each department has kept their records in their own file cabinets or computers. This, however, means that business data is fragmented and inter-departmental communication is far from seamless. Decision-making takes longer and the overall efficiency of the company is reduced.
Collaborative, cloud-based software such as modern MRP systems or even online spreadsheets like Google Sheets allow the necessary parties to have instant access to the same data. While building and managing a spreadsheet-based system becomes increasingly complex with the growing complexity of the business, the best ERP/MRP systems have been developed with scalability in mind.
With this readily-available data, you can instantly access data from across the business, which would substantially expedite decision-making and auditing processes, improve inventory management, and increase the overall efficiency of the company.
Key takeaways
- A batch record is the collection of data related to the manufacturing of a product batch, detailing the processing dates, the origin and quantities of the raw materials used, the staff involved, and the equipment utilized.
- These records ensure traceability in the supply chain and are therefore essential in quality assurance and regulatory compliance.
- Even though batch records are mostly associated with process manufacturing (e.g. pharmaceutical, biotech, food, and chemical industries), discrete manufacturing companies that produce in series can also benefit from the added transparency they provide.
- An electronic batch record is the product documentation created by a manufacturing software with traceability functionality.
- This virtual paper trail is the basis for identifying the root causes of issues and complying with modern traceability requirements as every product non-conformity issue can easily be traced back to specific stock lots, workstations, or employees.
- When keeping batch records, manufacturers need to keep in mind that: a) the more information they record, the better their batch records; b) data accuracy is key to regulatory compliance and an efficient company; c) one of the best ways to organize your business data and create batch records is by using an ERP/MRP system.
You may also like: Inventory Tracking – An Essential Guide for SMEs




